Tecovirimat (TPOXX)
People with monkeypox must not be left to suffer in pain while an approved drug sits on the shelf unused - Jay K. Varma & Joseph Osmundson
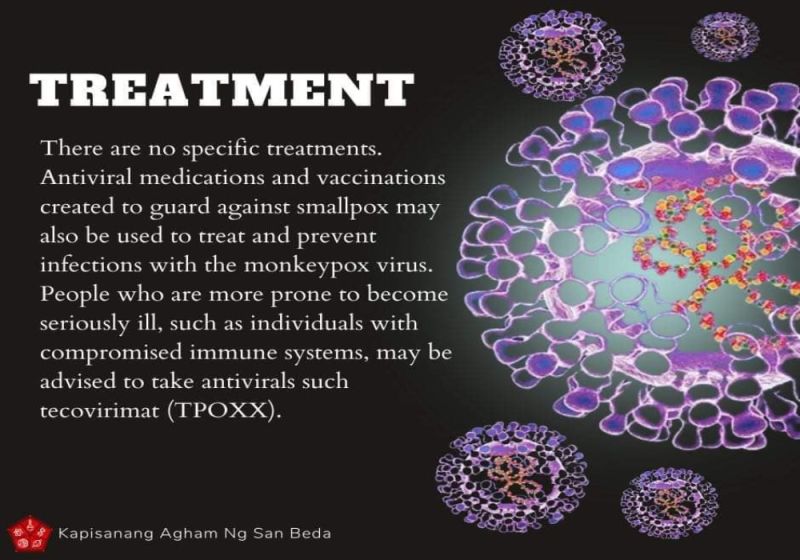
image by: Kapisanang Agham ng San Beda
HWN Recommends
Monkeypox patients should not be left to suffer when an FDA-approved drug could help
We believe there is an urgent need for the federal government to simultaneously align two public health objectives: make it easier for people with monkeypox to get tecovirimat and collect high-quality data on its effectiveness.
It is important to understand the history of tecovirimat, because it represents the impressive success of U.S. government pandemic preparedness and its simultaneous failure at pandemic response.
Resources
Drug to Treat Smallpox Approved by F.D.A., a Move Against Bioterrorism
The antiviral pill, tecovirimat, also known as Tpoxx, has never been tested in humans with smallpox because the disease was declared eradicated in 1980, three years after the last known case. But it was very effective at protecting animals deliberately infected with monkeypox and rabbitpox, two related diseases that can be lethal. It also caused no severe side effects when safety-tested in 359 healthy human volunteers, the F.D.A. said.
Information for Healthcare Providers on Obtaining and Using TPOXX (Tecovirimat) for Treatment of Monkeypox
Tecovirimat (also known as TPOXX or ST-246) is FDA-approved for the treatment of human smallpox disease caused by Variola virus in adults and children. However, its use for other orthopoxvirus infections, including monkeypox, is not approved by the FDA.
An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications
Tecovirimat showed protective efficacy against lethal challenge with every orthopoxvirus tested, in multiple animal models.
Can a smallpox drug treat monkeypox? Here’s what scientists know
Tecovirimat, a little-known antiviral, shows promise against monkeypox. But human data and supplies of the drug are limited.
Does a Smallpox Drug Work for Monkeypox? What Scientists Know
The antiviral Tecovirimat (TPOXX) shows promise against monkeypox, but human data and supplies are limited.
Getting monkeypox treatment is easier, but still daunting and confusing
The government's strategic national stockpile holds 1.7 million TPOXX courses. So far, 10,000 courses have been sent to states and cities on request – but "the number of [courses] deployed does not necessarily equate the number of [courses] administered," a spokesperson from the Department of Health and Human Services wrote in an email to NPR.
Slow response to monkeypox exposes ‘tired, overworked’ US health agencies
Virus has taken hold amid ragged system ravaged by years of underfunding, messy bureaucracy and Covid.
The most advanced treatments for monkeypox were developed to address the wrong threat
The markets may be in free fall, but a few biotechnology companies had an exceptionally good day. Siga’s stock is up more than 40%; Emergent Biosolutions’s gained nearly 12%, and Tonix Pharmaceuticals, 15%. Outside the US, Danish company Bavarian Nordic jumped 19%. The reason? They make treatments for monkeypox.
There Is an Effective Treatment for Monkeypox, but It’s Hard to Get
A smallpox antiviral that’s effective against monkeypox is tied up in red tape, and gay-health advocates are pushing to make it easier to access
TPOXX Smallpox Monkeypox Treatment
Clinicians and healthcare facility pharmacists can request TPOXX preferably by contacting their state or territorial health department; however, they can also reach out directly to CDC by contacting its Emergency Operations Center (EOC, 770-488-7100; [email protected]).
 Monkeypox patients should not be left to suffer when an FDA-approved drug could help
Monkeypox patients should not be left to suffer when an FDA-approved drug could help
Currently, most people with monkeypox have no access to this drug, and few providers are even aware it is available. Members of the gay community have been messaging each other to try to find it and are growing increasingly angry that they cannot access the one drug that could ease the suffering of people with monkeypox, not to mention their limited access to testing or vaccines.
TPOXX Dose
It is recommended that patients 13 kg and above initiate oral treatment with TPOXX capsules if possible. If patients are unable to take oral TPOXX capsules or Drug-Food Preparation, treatment may be initiated with TPOXX injection...

Introducing Stitches!
Your Path to Meaningful Connections in the World of Health and Medicine
Connect, Collaborate, and Engage!
Coming Soon - Stitches, the innovative chat app from the creators of HWN. Join meaningful conversations on health and medical topics. Share text, images, and videos seamlessly. Connect directly within HWN's topic pages and articles.