Adverse Drug Events
Did you know that the majority of FDA approved drugs have serious potential side effects that were not detected before marketing approval - Leo Galland MD
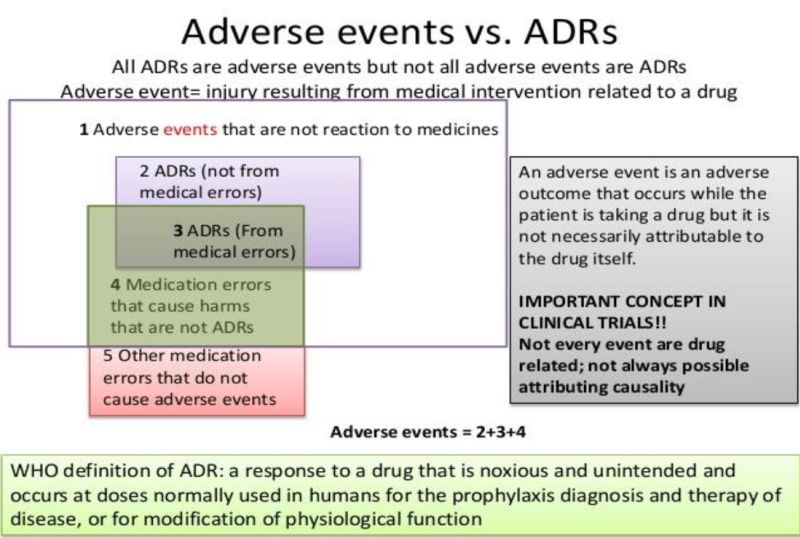
image by: Ijpsr Journal
HWN Suggests
Working together to prevent adverse drug events
In clinical practice, adverse drug events are often not documented in medical records, and not communicated between care providers and across healthcare sectors electronically. Our health care system currently relies heavily on patients and families to communicate adverse drug event information among their care providers. If adverse drug event information is shared directly between health care providers, it is largely communicated using paper, fax or phone. As a result, information often falls through the cracks, and care providers may unintentionally re-expose patients to culprit medications; medications that previously caused clinically significant adverse drug events or were previously…
Resources
 FDA Adverse Event Reporting System (FAERS)
FDA Adverse Event Reporting System (FAERS)
The FAERS Public Dashboard is a highly interactive web-based tool that will allow for the querying of FAERS data in a user friendly fashion. The intention of this tool is to expand access of FAERS data to the general public to search for information related to human adverse events reported to the FDA by the pharmaceutical industry, healthcare providers and consumers.
 MedWatch
MedWatch
MedWatch, the FDA’s medical product safety reporting program for health professionals, patients and consumers.
 One-Third Of New Drugs Had Safety Problems After FDA Approval
One-Third Of New Drugs Had Safety Problems After FDA Approval
Drugs ushered through the FDA's accelerated approval process were among those that had higher rates of safety interventions. These approvals typically rely on surrogate endpoints, meaning that researchers measured something other than survival, such as tumor size, to determine whether the drugs worked.
 What Americans Don’t Know About Their Medications
What Americans Don’t Know About Their Medications
People who have faced debilitating side effects say we need better warnings on drugs. The FDA hasn’t been enthusiastic.
 What side effects? Problems with medicines may be vastly underreported to the FDA
What side effects? Problems with medicines may be vastly underreported to the FDA
It’s no secret that the many side effects caused by medicines do not get reported to the Food and Drug Administration, but a new report suggests the magnitude of underreporting is far greater than imagined.
Adverse Drug Events vs Adverse Drug Reaction
An Adverse Drug Event (ADE) is “Harm caused by appropriate or inappropriate use of a drug whereas adverse drug reactions are a subset of these events, where harm is directly caused by a drug under appropriate use (i.e. at normal doses). Adverse drug events may include cases of provider error, non-adherence, or incorrect dosages.
How tech is tackling adverse drug events
The complexity of using EMRs and the risk of adverse drug events (ADE) globally can make it even harder to deliver the best patient care. The problem is getting worse as patients today are presenting with ever-increasing degrees of complexity, co-morbidities, and medication regimes.
National Action Plan for Adverse Drug Event Prevention
The ADE Action Plan identifies efforts to date to measure and prevent ADEs, and promote medication safety. In addition, this plan outlines future opportunities to advance patient safety with regard to the prevention of adverse drug events among three primary drug classes: anticoagulants, diabetes agents, and opioids.
Older patients on medley of drugs ‘at higher risk of adverse reactions’
Older women are at higher risk than older men of experiencing adverse reactions to drugs prescribed by their family doctor, and older patients taking more than 10 medicines are at higher risk than those taking fewer, according to a study.
Why Adverse Drug Events Occur and How to Prevent Them
An adverse drug event (ADE), according to the Office of Disease Prevention and Health Promotion, is “an injury resulting from medical intervention related to a drug. This includes medication errors, adverse drug reactions, allergic reactions, and overdoses.”
Why Adverse Drug Events Occur and How to Prevent Them
Adverse drug event (ADE) is an umbrella term that refers to any type of unexpected, potentially harmful event that happens after you take a medication, whether it be during appropriate or inappropriate circumstances.
 Working together to prevent adverse drug events
Working together to prevent adverse drug events
Adverse drug events, the harmful and unintended consequences of medication use, are a leading cause of unplanned hospital admissions, and rank between the 4th and 6th cause of death in North America.
Action ADE
Working together to prevent adverse drug events. ActionADE is a research project focused on preventing the "adverse drug events" (ADEs) that happen when medications have harmful and unintended consequences. The ActionADE project is part of our larger Adverse Drug Event Research Program.
CDC
Although antibiotics are good drugs for certain types of infections, they are also one of the types of medicines that cause the most emergency visits for adverse drug events.

Introducing Stitches!
Your Path to Meaningful Connections in the World of Health and Medicine
Connect, Collaborate, and Engage!
Coming Soon - Stitches, the innovative chat app from the creators of HWN. Join meaningful conversations on health and medical topics. Share text, images, and videos seamlessly. Connect directly within HWN's topic pages and articles.